Chemically Modified AMPA Receptor RNA Aptamers Designed for In Vivo Use
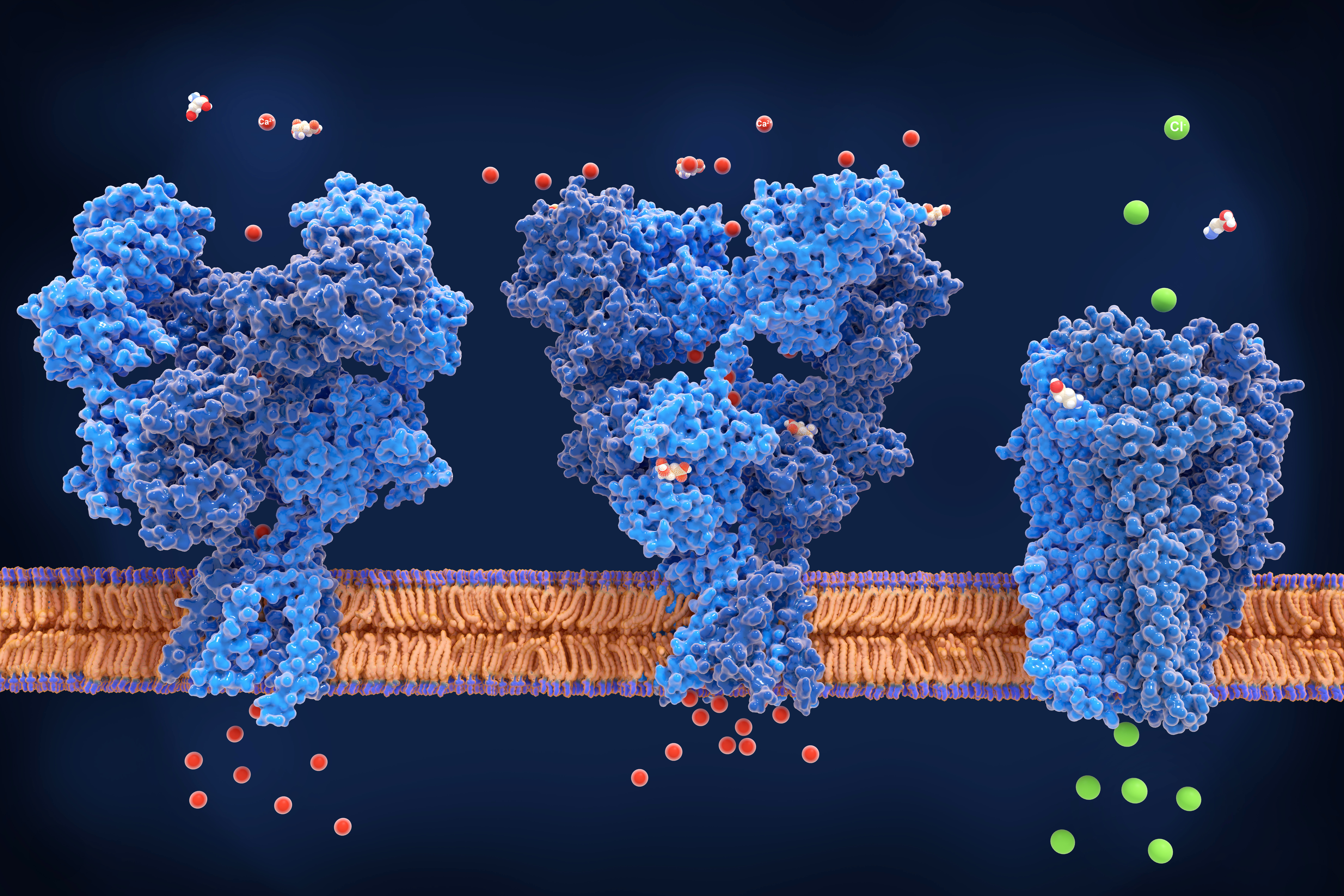
Neurological disorders are increasingly recognized as major causes of death and disability worldwide. Nearly 500,000 cases of brain tumor, multiple sclerosis, and amyotrophic lateral sclerosis (ALS) are diagnosed each year. Further, there are about 135 million epilepsy episodes annually and more than 10 million people worldwide are living with Parkinson’s disease. Glutamate ion channels have three subtypes: AMPA, kainate, and NMDA receptors. Excessive activity of these receptor subtypes either individually or collectively is associated with various neurological disorders such as ALS, Huntington’s disease, epilepsy, etc. To date, small-molecule compounds are the dominating drug candidates in the field. However, lower potency, cross activity and poor water solubility are generally associated with these compounds. In clinical settings, these compounds have shown significant adverse effects, which have prevented their use as potential drug candidates. RNA aptamers are a novel group of antagonists with superior pharmacological properties to traditional, small molecule compounds. These RNA aptamers are potential therapeutics for a range of neurological disorders.
This technology, developed by University at Albany researchers, involves the isolation of two important aptamers that can be useful in the treatment of various neurological disorders. The short aptamer, FN1040s selectively inhibits the GluA1 and GluA2Qflip AMPA receptor subunits. The full-length aptamer FN1040 additionally inhibits GluK1, but not GluK2, kainate receptor, and GluN1a/2A and GluN1a/2B (the two major native NMDA receptors). The two aptamers show similar potency (2-4 REPLACE) and are stable with a half-life of at least 2 days in serum-containing medium or cerebrospinal fluid which makes them candidates for in vivo use. Because these aptamers exhibit higher potency and selectivity towards AMPA receptors than current small molecule compounds, they offer an alternative approach for developing therapies. Further, because aptamers are RNA molecules, they are naturally water soluble and thus more bioavailable, allowing for lower dose administration.
 Inpart Image source
Inpart Image source
These RNA aptamers offer a new approach to treatment of neurological disorders and present compelling advantages such as: High potency rivaling existing small molecule inhibitors. Excellent selectivity. Better water solubility compared to other approaches.
The RNA aptamers described here could be used to develop therapies for a range of neurological disorders such as ALS, Huntington’s disease, and epilepsy.
Patented
U.S. patent 20190010498 for “Chemically modified AMPA receptor RNA aptamers” was issued on Jan 10, 2019.
This technology is available for licensing.
Development partner - Commercial partner - Licensing
Additional Information:
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
Patent Status |
File Date |
Issued Date |
Expire Date |
|