A nanoparticle-based method for enhanced delivery of protein/peptide therapeutics.
Biologics, for example protein and peptide-based therapeutics, have poor in vitro shelf stability and in vivo enzymatic stability, which lead to short blood circulating time and contribute to immunogenicity. In addition, biologics are impermeable to biological barriers with poor bioavailability and cell uptake. Encapsulation of active protein inside a nanoparticle can increase stability and improve efficacy for better disease treatment in patients. Unfortunately, therapies of this type are limited by the lack of efficient carriers that improve in vivo stability, reduce immunogenicity, and can deliver the therapeutic into intracellular space while maintaining bioactivity. The goal is to create small nanoparticles (10 to 30 nm) with high protein loading ability and cell-penetration properties. However, it remains technically challenging to create particles of this size while maintaining protein loading capacity.
This technology is a novel method for protein drug delivery. The technology consists of functional segregated telodendrimers that can have two or three functional segments. The telodendrimers consist of a linear polyethylene glycol and a dendritic polylysine/polyarginine with flexible linker-conjugated hydrophobic natural compounds. These telodendrimers can associate with the protein surface for effective protein coating and encapsulation into neutral and stable sub-30 nm nanoparticles with high amounts of proteins (30 to 200% of the telodendrimer by weight). This is capable of intracellular protein delivery. The favorable physical properties and biocompatibility of the telodendrimer nanoparticles make them highly suitable as nanocarriers for protein-based therapies, such as antibodies and insulin for cancer and diabetes treatments.
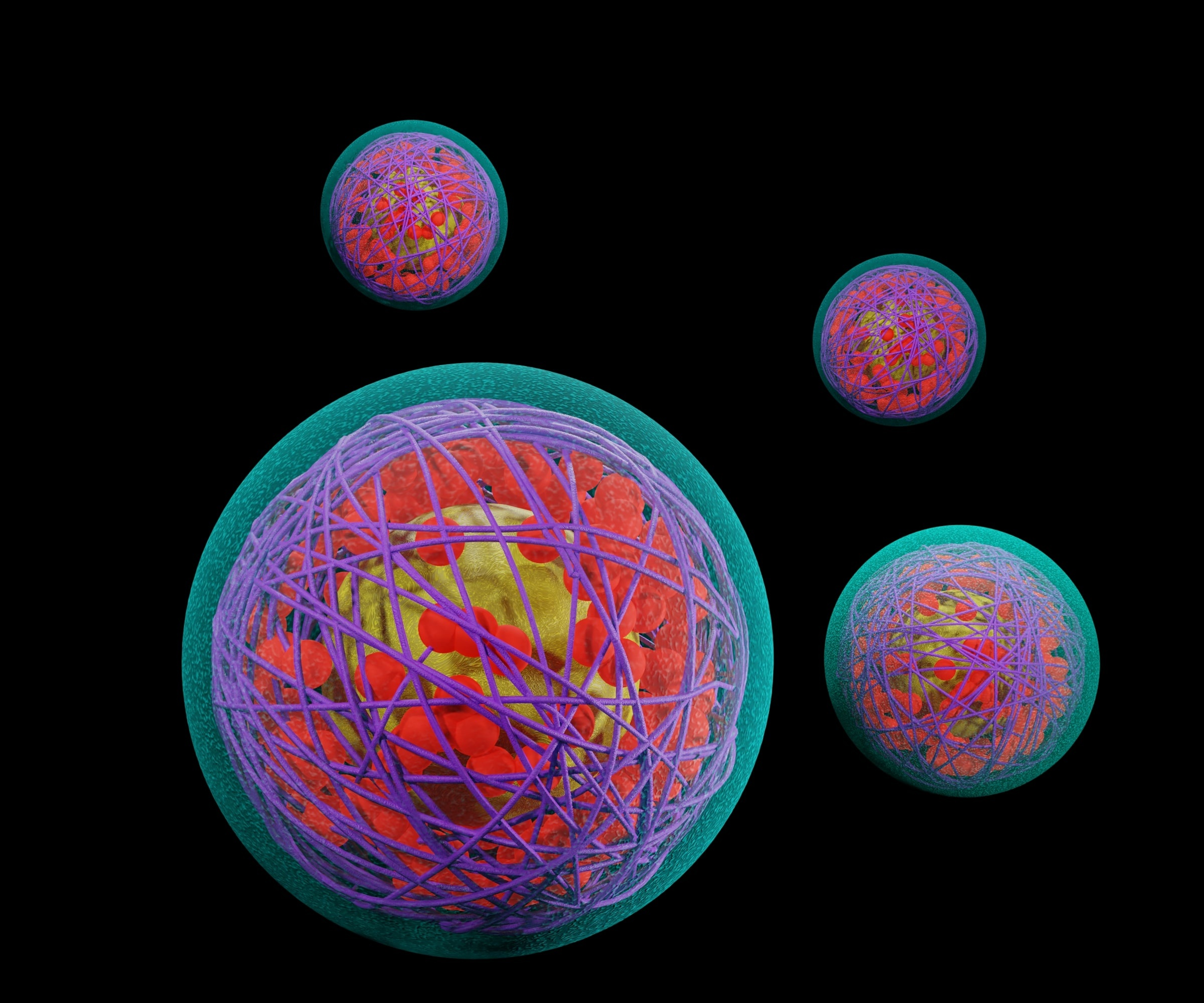
• In situ protein loading.
• No organic solvent and adjuvant free.
• High loading capabilities.
• Enhanced bioavailability with reduced cytotoxicity.
• Size, capacity, and cell penetration can be adjusted.
• Improved in vivo stability.
• Better control over protein release.
• Well-defined chemical structure.
• Improved passive tumor targeting.
The primary application for this technology is protein/peptide encapsulation and delivery.
Granted Patents: US10,947,350, EP3347053
TRL 3 - Experimental proof of concept
This technology is available for licensing
This technology would be of interest to anyone involved in the development of carrier-based therapeutics, including:
• Pharmaceutical companies.
• Hospitals.
• Medical research laboratories.
• Educational institutions.